Interaction of Pool Water and Air Chemistry | TB09
With the acceptance of ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers) Standard 62.1-2022 concerning indoor air quality, more stringent reviews are required of an indoor pool’s air quality. This technical bulletin summarizes the chemistry involved for pool water using chlorine and its effects on air quality, and vice versa.
Gated Content Overview Headline
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Gated Content
Please fill out the form for access.
Overview
With the acceptance of ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers) Standard 62.1-2022 concerning indoor air quality, more stringent reviews are required of an indoor pool’s air quality. This technical bulletin summarizes the chemistry involved for pool water using chlorine and its effects on air quality, and vice versa.
Introduction
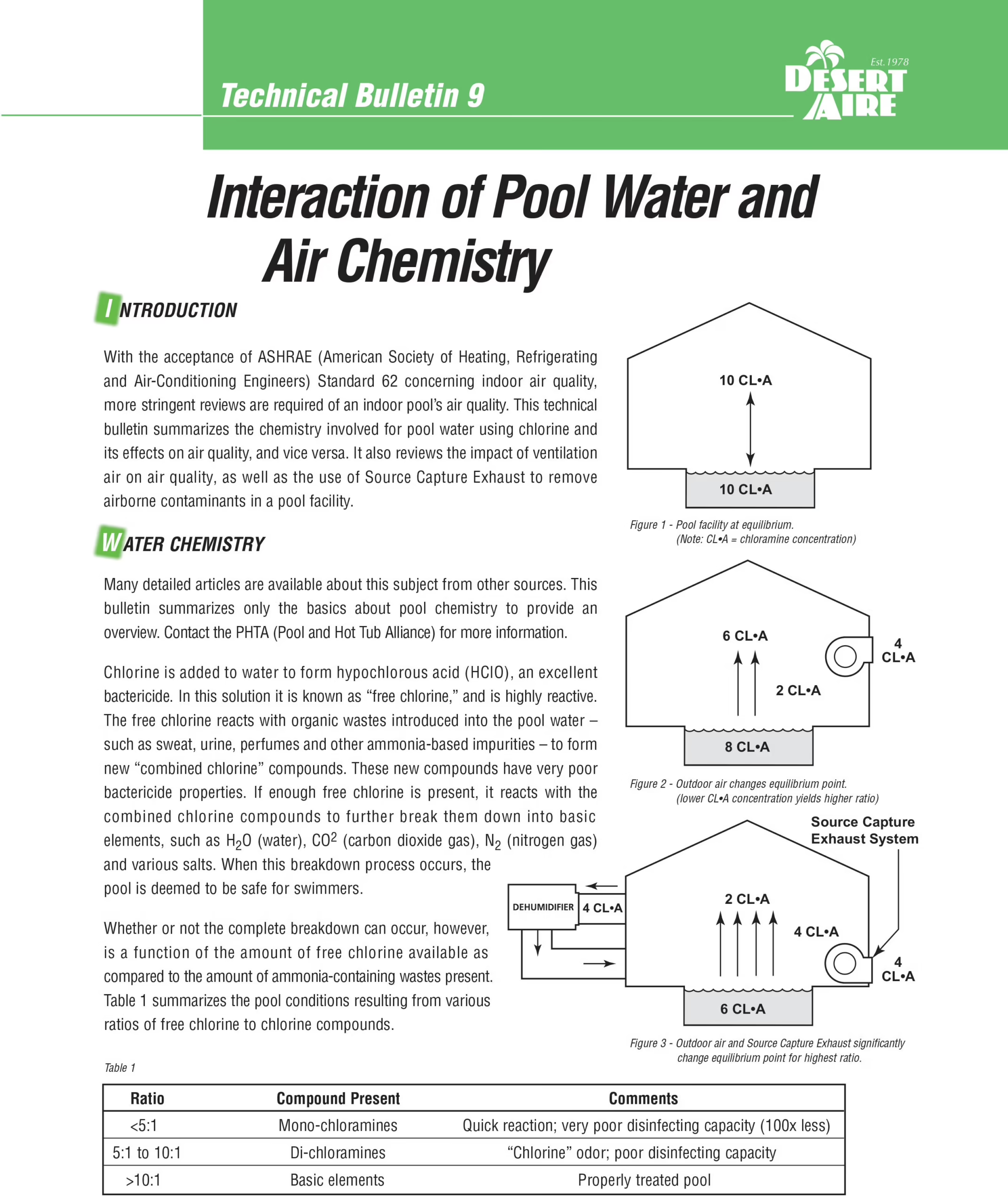
With the acceptance of ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers) Standard 62 concerning indoor air quality, more stringent reviews are required of an indoor pool’s air quality. This technical bulletin summarizes the chemistry involved for pool water using chlorine and its effects on air quality, and vice versa. It also reviews the impact of ventilation air on air quality, as well as the use of Source Capture Exhaust to remove airborne contaminants in a pool facility.
Water Chemistry
Many detailed articles are available about this subject from other sources. This bulletin summarizes only the basics about pool chemistry to provide an overview. Contact the PHTA (Pool and Hot Tub Alliance) for more information.
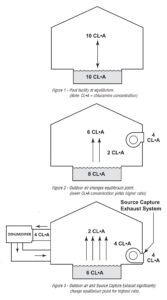
Chlorine is added to water to form hypochlorous acid (HClO), an excellent bactericide. In this solution it is known as “free chlorine,” and is highly reactive. The free chlorine reacts with organic wastes introduced into the pool water – such as sweat, urine, perfumes and other ammonia-based impurities – to form new “combined chlorine” compounds. These new compounds have very poor bactericide properties. If enough free chlorine is present, it reacts with the combined chlorine compounds to further break them down into basic elements, such as H2O (water), CO2 (carbon dioxide gas), N2 (nitrogen gas) and various salts. When this breakdown process occurs, the pool is deemed to be safe for swimmers.
Whether or not the complete breakdown can occur, however, is a function of the amount of free chlorine available as compared to the amount of ammonia-containing wastes present. Table 1 summarizes the pool conditions resulting from various ratios of free chlorine to chlorine compounds.
| Ratio | Compound Present | Comments |
|---|---|---|
| <5:1 | Mono-chloramines | Quick reaction; very poor disinfecting capacity (100x less) |
| 5:1 to 10:1 | Di-chloramines | “Chlorine” odor; poor disinfecting capacity |
| >10:1 | Basic elements | Properly treated pool |
As Table 1 shows, a constant source of free chlorine is needed to ensure the complete reaction. This is known as breakpoint chlorination. If the combined chlorine compounds are not eliminated, pool “shocking” is required: a larger dose of chlorine is added to the water to complete the reaction and balance the pool.
Air Chemistry
The pool room odor commonly described as “chlorine” (which, in fact, is the odor produced by chloramine compounds, a disinfectant by-product) occurs when the pool water chemistry is improperly balanced. The chloramines readily release into the air and reach a balance based on a chemical law known as the partial pressure law. In laymen’s terms, this law states how much chloramine remains in the water and how much is released to the air under various conditions.
The ASHRAE 62 ventilation standard recognizes this “chlorine” smell as a potential indoor air quality problem and offers specific recommendations for the introduction of outdoor air based on the size of the pool and deck. (Refer to Desert Aire’s Technical Bulletin 5 – Ventilation Air for Indoor Pools, for details on these recommendations.) The standard attempts to replace the indoor air once per hour to eliminate the odor.
Since nature requires a balance, removing some of the chloramines from the air will cause more chloramines to be released from the water. Table 1 shows that the release of more chloramines to the air will improve the free chlorine ratio, bringing the pool chemistry a step closer to proper balance.
More Outdoor Air
The response of some pool designers is to go beyond ASHRAE 62 outdoor air ventilation recommendations. That scenario, however, can introduce other problems.
First, in cold climates, wintertime outdoor air must be heated. For even the smallest pools, this adds up to thousands of dollars per month in increased utility bills.
Source Capture Exhaust
Source Capture Exhaust will assist with removing the airborne chloramines if designed properly. The airborne chloramines are 4X heavier than air and will accumulate above the pool water surface. Incorporating a source capture exhaust at the deck level has shown to dramatically improve the air quality. See Desert Aire’s Guide to an Integrated HVAC System Design for the 21st Century Natatorium for more details.
Conclusion
While this technical bulletin does not attempt to cover all chemistry issues (for example, the influence of pH on free chlorine), it does demonstrate the basic chemical interaction occurring in an indoor pool facility.
The following design specifications are recommended:
- Automatic chlorate control system. The chemical feed pump must be sized to match worst case pool loading.
- High water turnover to better mix the pool, to avoid dead spots, and to provide better chlorine concentration measurement and control.
- Ensure ASHRAE 62 outdoor air compliance to aid in breakpoint chlorination.
- Incorporate Source Capture Exhaust systems such as an Evacuator to improve air quality in high activity pool rooms.
Learn more in our blog: How 3 Key Dehumidification Terms Relate
Related Products

ExpertAire Horizontal (LC), Vertical (LV) & Packaged (LCQ) Series Dehumidifier
Designed for high efficiency and long life, Desert Aire’s ExpertAire™ commercial dehumidification ...
View Details

SwimAire™ Series Dehumidifier
With 39 to 112 lbs/hr of moisture removal capacity, 3,000 to 8,500cfm of air handling and 8 to 23 tons...
View Details

SelectAire™ Series Dehumidifier
The SelectAire™ commercial dehumidification systems are ideal for large-scale moisture removal appli...
View Details

SelectAire Plus™ (SP) Series Dehumidifier
Designed specifically for large 50 meter pool applications, the SelectAire Plus™ Series modular dehu...
View Details
Find a Desert Aire Sales Rep Near You!
Our network of independent representatives are fully trained on Desert Aire’s dehumidification and DOAS solutions and can assist you in designing and sizing your engineered solutions.

